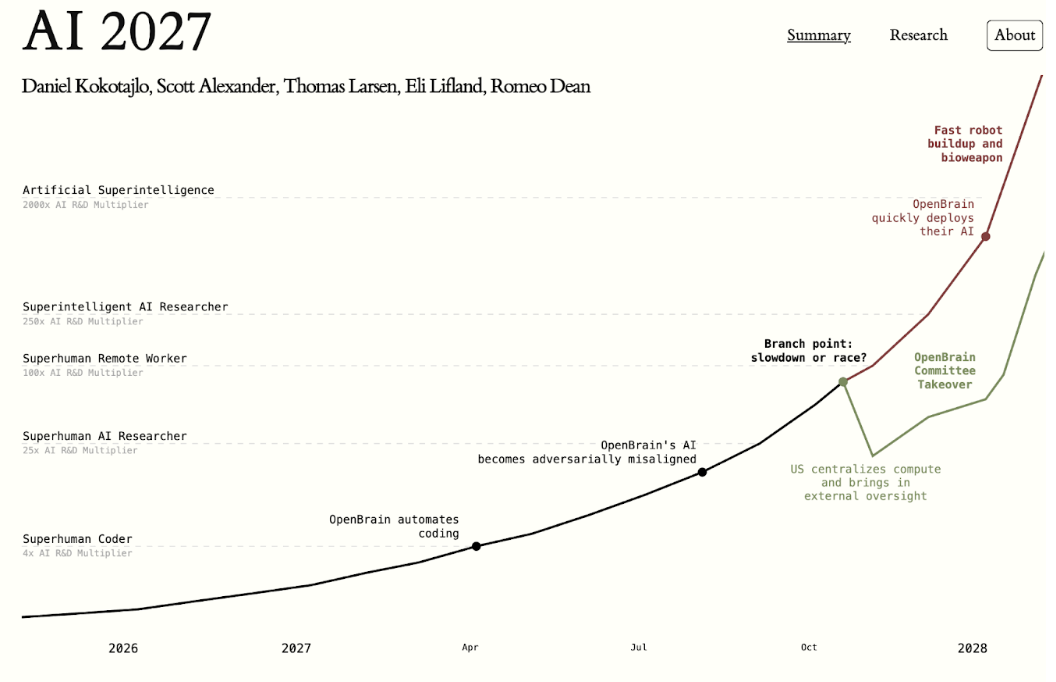
Kyle and Nicole Muldoon had their baby boy KJ Muldoon in August of 2024 at the Children’s Hospital of Philadelphia, but they soon noticed that something wasn’t quite right. One week after his birth, the doctors discovered that KJ had a rare genetic mutation – a CPS1 deficiency – which affects only one in 1.3 million babies born. With this disorder, little KJ’s body couldn’t process nitrogen, which led to the buildup of ammonia in his body. Half of these babies die within the first week of birth because the deficiency goes undetected, but those who survive suffer severe developmental delays and need a liver transplant later in life. Even though these delays sacrificed both KJ’s life expectancy and his quality of life, his parents wanted to explore their options of treatment.
Although the chance of survival was grim and would lead to a life of extensive treatment, the Muldoons wanted to treat KJ to give him a chance at life. Instead of the traditional treatment, which involves countless medications and an adjusted low-protein diet, the doctors used CRISPR gene-editing technology to correct the mutation causing KJ’s deficiency. CRISPR works by using a guide RNA to locate a specific target DNA sequence and then using a protein called Cas9 to cut the DNA at that location. Then, in this cut, the desired CPS1 that KJ lacked was placed in his genetic code. This gene-editing treatment was administered through an infusion, and KJ is now a happy, healthy nine-month-old baby.
Although KJ’s recovery was miraculous, his treatment meant more than that, as he marked the beginning of personalized DNA treatment. With the technology that KJ’s doctors used, hundreds of thousands of patients with rare genetic disorders have a possibility of being cured, something that they hadn’t even fathomed until recently.
However, there are some debates about the use of genetic therapy and gene editing in general. Many believe that the idea of gene editing is a slippery slope, even when it is deemed a medical necessity. These people fear that a rise in gene editing will mimic the rise of eugenics in the 1920s, which believed in “perfecting” the human race. If we continue to make everyone healthy and eradicate diseases and deficiencies, then are we “perfecting” humans?
For others, gene editing is a medical miracle that would work wonders in curing previously incurable diseases. Nobody wants their baby to be sick or die from a disease, and the implementation of gene editing technology like the kind that saved KJ could save many others suffering from genetic mutations in the future.
But where do we draw the line? For students like HHS junior Anne Kyriss, gene editing, although “a great step forward in the medical field, has a downfall because it opens up to what genes are considered valuable.” On the other hand, students like freshman Madeleine Sweeney highlight the value of introducing personalized gene therapy. She told the Harborlight, “The fact that this new technology saved so many lives should be celebrated. It opens so many possibilities for the future care of rare genetic disorders.” Both sides of the argument on gene therapy are incredibly valid, and both must be taken into consideration.
If you ask me, I think that genetic editing for babies like KJ is a step in the right direction, as long as genetic editing is strictly used for medical necessity. There were many things that KJ wouldn’t have been able to do with his deficiency, and it also would have shortened his lifespan dramatically. However, if parents wanted to edit their baby’s genes to give them blue eyes or grow taller, that should 100% be prohibited. Gene therapy is an extremely powerful technology, but with great power comes great responsibility. Gene editing will save lives and should be used as long as strict regulation is used to provide strictly medical gene therapy for individuals with genetic mutations.